studied Biochemistry at the MLU in Halle where he also obtained his PhD in 2018. Since 2018, he works as a postdoctoral researcher in the group of Andrea Sinz at the Faculty of Natural Sciences I of the MLU. Currently, he conducts research on the role of intrinsic disorder for DNA-binding of the tumor suppressor p53.
Project within the RTG
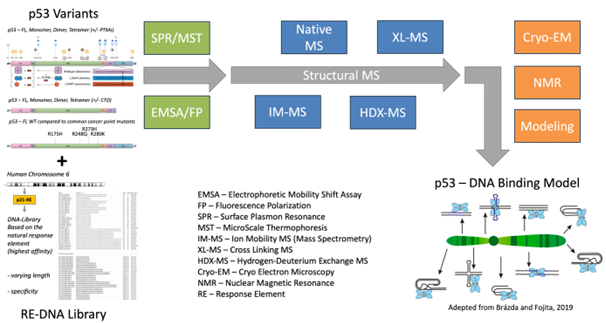
The N- and C-terminal region of the full-length p53 tetramer are still poorly understood due to their high degree of disorder but are thought to be heavily involved in the DNA binding process. A combination of DNA-binding assays and methods for protein structure elucidation followed by a computational modeling approach will help to elucidate the p53 DNA – recognition and -binding mechanism.
Literature references
Di Ianni, A., Tüting, C., Kipping, M., Ihling, C. H., Köppen, J., Iacobucci, C., Arlt C.*, Kastritis P. L., Sinz A.* (2023). Structural Assessment of the Full-Length Wild-Type Tumor Suppressor Protein p53 by Mass Spectrometry-Guided Computational Modeling. Sci Rep., 13(1): 8497.
Arlt, C., Flegler, V., Ihling, C. H., Schäfer, M., Thondorf, I., Sinz, A.* (2017). An Integrated Mass Spectrometry-Based Approach to Probe the Structure of the Full-Length Wild-Type Tetrameric p53 Tumor Suppressor. Angew Chem Int Ed Engl., 56(1): 275-279.
Arlt, C., Ihling, C. H., Sinz, A.* (2015). Structure of full-length p53 tumor suppressor probed by chemical cross-linking and mass spectrometry. Proteomics., 15(16): 2746-55.
Website: http://agsinz.pharmazie.uni-halle.de